
Attenuated Total Reflectance (ATR)
$99 Base price View My Quote RequestPorosimetry
Porosimetry (also called “Gas Adsorption” or “Physisorption” Analysis or “BET Analysis”) is a highly flexible and accurate technique for measuring specific surface area, pore sizes / size distribution, and overall porosity of solid samples as well as active metal area, dispersion and crystallite size for materials with gas-reactive surface like catalysts. These can impact electrical, physical, and thermal properties and affect overall performance of devices and systems.
See also:
Capillary Flow Porometry (or Porometry) is an alternative porosity measurement technique suited for permeable samples.
- Efficient characterization of solid sample surface area, porosity, pore size distribution, active metal area, dispersion and crystallite size
- Highly accurate, detailed, and reproducible
- Accommodates high-throughput analysis
- Automated sample preparation to maximize precision
- Not optimized for large pore sizes (greater than 0.5 μm)
Technical Specifications:
Learn More:
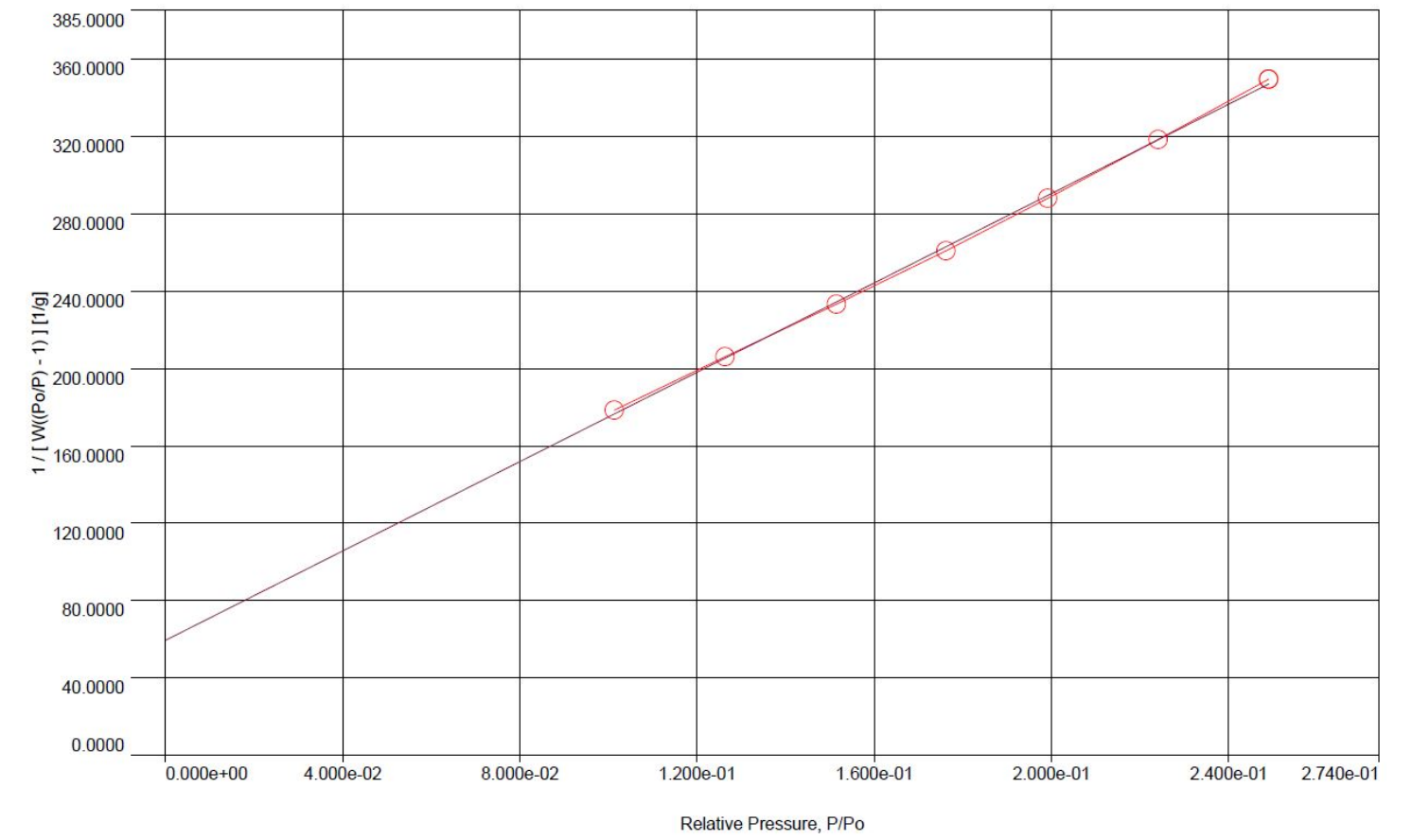
Example BET Surface Area plot measured with the Anton Paar Autosorb iQ sorption analyzer. This plot shows one of two powder samples analyzed with a mean surface area of 2.59 m2 / g.

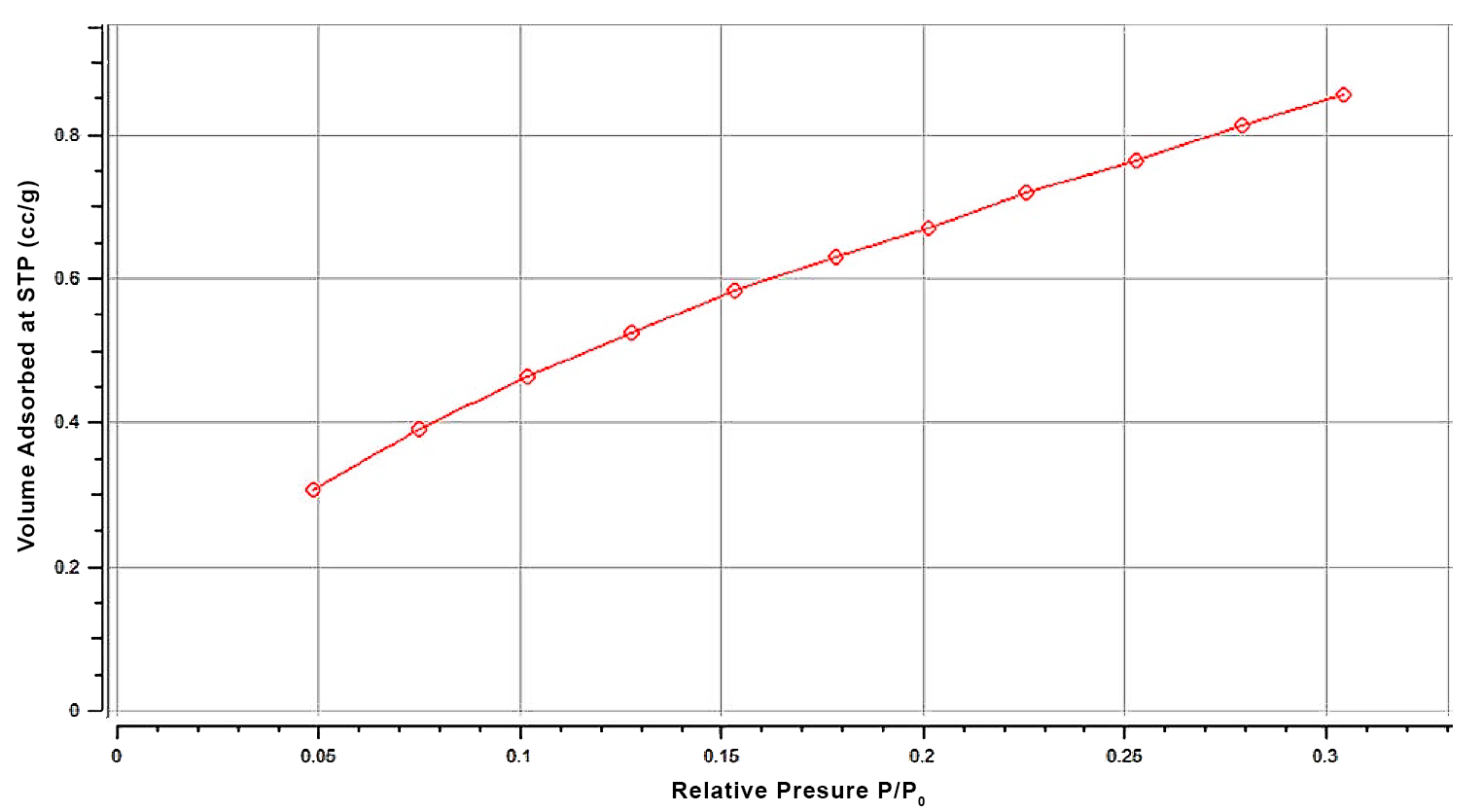
Example multi-point isotherm for Magnesium Stearate collected at 11 different pressures using the Anton Paar NOVAtouch system. This isotherm is used to compute the BET specific surface area for the material.

Anton Paar Autosorb iQ
- Pressure Range: 0.1 torr + 10 torr + 1000 torr
- Pressure Accuracy:
- 1 torr, 1.0 torr: < ± 0.15 % of reading
- 10 torr: < ± 0.12 % of reading
- 1000 torr: < ± 0.11 % of reading
- Minimum Detectable Pore Size: 0.35 nm
- Maximum Detectable Pore Size: 500 nm
- Minimum Detectable Surface Area: 0.0005 m2 / g
(with Kr adsorbate at Liquid Nitrogen Temperature) - Ultimate Vacuum Pressure: 5 x 10-10 mbar
- Adsorbate Species: N2, Ar, Kr, CO2, O2, H2, CH4, and more

Anton Paar NOVA 800
- Full isotherm collection
- Pressure Range: 0 torr to 1000 torr
- Pressure Resolution: 0.00006 torr (60 mtorr)
- Pressure Accuracy: within ± 0.1% of span
- Surface Area Range: 0.01 m2/g to no known upper limit
- Pore Volume Limit (STP): < 0.0001 cc/g
- Pore Volume Limit (liquid): < 2.2 x 10-6 ccMinimum Detectable Pore Size: 0.35 nm
- Maximum Detectable Pore Size: 500 nm
- Reproducibility: < 2%
- Adsorbate Species: N2, Ar, Kr, CO2, and any other inert gas
Porosity describes the total volume of empty pockets of space on solid surface and air (voids) enclosed within solid materials. These voids are often critically important in affecting the thermal, electrical, and mechanical performance of both raw materials and engineered parts and products.
Covalent can perform Porosity analysis with these techniques:
- Gas Adsorption / Physisorption
- Chemical Gas Adsorption (see below)
- Capillary Flow Porometry
Physisorption analysis uses the Brunauer-Emmett-Teller (BET) theory, which provides a mathematical model for quantifying the adsorption of gas molecules into a solid surface area. How many molecules are adsorbed will depend on the temperature and pressure of the gas (which are controlled), as well as the surface energy distribution, surface area, and porosity of the sample (which are analyzed).
To make the measurement, a sample is loaded into a closed environment and flushed with an inert gas – most commonly: Nitrogen. The sample is then cooled to liquid-nitrogen temperature. This causes the gas molecules to adhere to the sample surface via weak solid-gas interactions.
When the cooling is removed, these molecules desorb and can be quantified to characterize the total sample surface: including porosity (total pore volume), pore volume distribution, and pore size distribution.
Chemisorption, or Chemical Gas Adsorption, is an alternative type of adsorption in which a chemical reaction occurs between the sample and adsorbed gas particles. This technique is especially useful for analyzing catalysts, batteries, and other activated porous systems and samples, or in studying corrosion in metals. The method of this technique is similar to physisorption; however the isotherm produced more specifically probes only the chemically active areas where bonds are formed.

4 Targets for Optimization in a Battery Cell

Batteries Industry Solutions
Porous Materials Characterization with Porometry, Porosimetry, and Pycnometry

Graphene Characterization

Analysis of PTFE: Why does it seal well?

Pore Size and Pore Volume Measurement of Metal...

Surface Area of Magnesium Stearate Using the Volumetric...
Capillary Flow Porometry (Porometry)
Capillary Flow Porometry (also called Porometry) is an optimal technique for characterizing through-pore size and size distribution.

